人类和其他高级灵长类动物的痛风是自发形成的。人类不再表达尿酸酶基因,而动物体内尿酸更多被降解为可溶性复方尿囊素。这一因素加上肾脏对尿酸盐的高速再吸收可导致高尿酸血症和痛风。[13]Johnson RJ, Rideout BA. Uric acid and diet - insights into the epidemic of cardiovascular disease. N Engl J Med. 2004;350:1071-1073.http://www.ncbi.nlm.nih.gov/pubmed/15014177?tool=bestpractice.com[14]Wu XW, Lee CC, Muzny DM, et al. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci USA. 1989;86:9412-9416.http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=2594778http://www.ncbi.nlm.nih.gov/pubmed/2594778?tool=bestpractice.com[15]Wu XW, Muzny DM, Lee CC, et al. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78-84.http://www.ncbi.nlm.nih.gov/pubmed/1556746?tool=bestpractice.com[16]Richette P, Bardin T. Gout. Lancet. 2010;375:318-328.http://www.ncbi.nlm.nih.gov/pubmed/19692116?tool=bestpractice.com 在生理条件下,尿酸以尿酸盐形式存在。高尿酸盐水平导致过饱和、形成结晶,进而导致痛风。尿酸盐水平与患病风险直接相关。降低尿酸盐水平的药物可降低疾病反复发作的风险。[17]Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51:321-325.http://www3.interscience.wiley.com/cgi-bin/fulltext/109062089/HTMLSTARThttp://www.ncbi.nlm.nih.gov/pubmed/15188314?tool=bestpractice.com
关节内尿酸盐的溶解度取决于温度、pH 值、非聚集蛋白聚糖及其他因素。痛风通常会影响第一跖趾关节(身体较冷部位)和骨关节炎好发关节。[18]Fam AG, Stein J, Rubenstein J. Gouty arthritis in nodal osteoarthritis. J Rheumatol. 1996;23:684-689.http://www.ncbi.nlm.nih.gov/pubmed/8730127?tool=bestpractice.com
关节内的尿酸盐结晶与未分化的吞噬细胞相互作用,并通过诱导 TNF-α 和激活信号通路和内皮细胞引起急性炎症反应。[19]Yagnik DR, Hillyer P, Marshall D, et al. Noninflammatory phagocytosis of monosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout. Arthritis Rheum. 2000;43:1779-1789.http://www3.interscience.wiley.com/cgi-bin/fulltext/78502428/PDFSTARThttp://www.ncbi.nlm.nih.gov/pubmed/10943868?tool=bestpractice.com TNF-α、白介素 (IL)-8 和其他趋化因子导致中性粒细胞粘附内皮、汇集和扩增,进而导致中性粒细胞性滑膜炎。
秋水仙碱通过干扰中性粒细胞-内皮细胞之间的相互作用而发挥作用。[10]Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143:499-516.http://www.ncbi.nlm.nih.gov/pubmed/16204163?tool=bestpractice.com[20]Cronstein BN, Molad Y, Reibman J, et al. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96:994-1002.http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=7543498http://www.ncbi.nlm.nih.gov/pubmed/7543498?tool=bestpractice.com 在对尿酸结晶反应产生的中性粒细胞趋化作用方面,IL-8 的作用占 90%;因此,抑制 IL-8 可能具有治疗意义。[21]Terkeltaub R, Zachariae C, Santoro D, et al. Monocyte-derived neutrophil chemotactic factor/interleukin-8 is a potential mediator of crystal-induced inflammation. Arthritis Rheum. 1991;34:894-903.http://www.ncbi.nlm.nih.gov/pubmed/2059236?tool=bestpractice.com[22]Nishimura A, Akahoshi T, Takahashi M, et al. Attenuation of monosodium urate crystal-induced arthritis in rabbits by a neutralizing antibody against interleukin-8. J Leukoc Biol. 1997;62:444-449.http://www.ncbi.nlm.nih.gov/pubmed/9335313?tool=bestpractice.com 此外,有证据表明尿酸盐结晶可激活 NALP3 炎性复合体,该复合体可诱导 IL-1β 分泌,而 IL-1β 在痛风炎性反应中发挥作用。[23]Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237-241.http://www.ncbi.nlm.nih.gov/pubmed/16407889?tool=bestpractice.com 该途径已成为痛风治疗中治疗干预的新靶点,即抑制 IL-1β 反应。[24]Terkeltaub R, Schumacher HR Jr, Saag KG, et al. Evaluation of rilonacept for prevention of gout flares during initiation of urate-lowering therapy: results of a phase 3, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(suppl):152.http://www.blackwellpublishing.com/acrmeeting/abstract.asp?MeetingID=774&id=88807[25]Terkeltaub R, Schumacher HR Jr, Curtis C, et al. Evaluation of rilonacept in patients with gouty arthritis experiencing an acute gout attack. Arthritis Rheum. 2010;62(suppl):153.http://www.blackwellpublishing.com/acrmeeting/abstract.asp?MeetingID=774&id=88808[26]Schlesinger N, Lin HY, De Meulemeester M, et al. Efficacy of canakinumab (ACZ885), a fully human anti-interleukin (IL)-1beta monoclonal antibody, in the prevention of flares in gout patients initiating allopurinol therapy. Arthritis Rheum. 2010;62(suppl):2087.http://www.blackwellpublishing.com/acrmeeting/abstract.asp?MeetingID=774&id=90730[27]So A, De Meulemeester M, Pikhlak A, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62:3064-3076.http://www.ncbi.nlm.nih.gov/pubmed/20533546?tool=bestpractice.com
痛风发作的自发缓解因清除尿酸盐晶体所致,后者通过已分化的吞噬细胞、蛋白质包裹晶体、中性粒细胞凋亡和炎症介质灭活实现。[10]Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143:499-516.http://www.ncbi.nlm.nih.gov/pubmed/16204163?tool=bestpractice.com 尿酸盐晶体可能引起慢性炎症,进而导致滑膜炎、软骨流失和骨侵蚀。它们也可能诱导软骨细胞产生金属蛋白酶和一氧化氮,其可导致软骨流失,且可能通过抑制成骨细胞引起骨质破坏。[28]Liu R, Liote F, Rose DM, et al. Proline-rich tyrosine kinase 2 and Src kinase signaling transduce monosodium urate crystal-induced nitric oxide production and matrix metalloproteinase 3 expression in chondrocytes. Arthritis Rheum. 2004;50:247-258.http://www3.interscience.wiley.com/cgi-bin/fulltext/106600976/HTMLSTARThttp://www.ncbi.nlm.nih.gov/pubmed/14730623?tool=bestpractice.com[29]Bouchard L, de Medicis R, Lussier A, et al. Inflammatory microcrystals alter the functional phenotype of human osteoblast-like cells in vitro: synergism with IL-1 to overexpress cyclooxygenase-2. J Immunol. 2002;168:5310-5317.http://www.jimmunol.org/cgi/content/full/168/10/5310http://www.ncbi.nlm.nih.gov/pubmed/11994489?tool=bestpractice.com
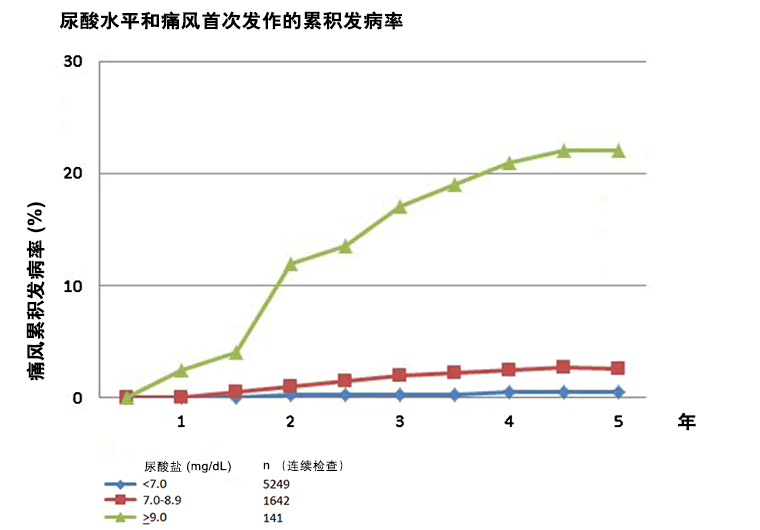 [Figure caption and citation for the preceding image starts]: 尿酸水平和痛风首次发作的累积发病率改编自 Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421-426 [Citation ends].
[Figure caption and citation for the preceding image starts]: 尿酸水平和痛风首次发作的累积发病率改编自 Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421-426 [Citation ends].